
Likewise, 1.0 mole of H2Ois composed of 2.0 moles of hydrogenand 1.0 mole of oxygen. Thus, H2O is composed of two atoms of hydrogen and 1 atom of oxygen. A simple example of this concept is that the empirical formula of sulfur monoxide or SO would simply be SO as is the empirical formula of disulfur dioxide S 2 O 2. The ratios hold true on the molarlevel as well. In chemistry the empirical formula of a chemical compound is the simplest whole number ratio of atoms present in a compound. so you need to multiply the numbers in the empirical formula by 3 An empirical formula tells us the relative ratios of different atoms in a compound.the mass of the atoms in the empirical formula is 14.Converting the empirical formula to a molecular formulaįrom the empirical formula, you can work out the molecular formula if you know the relative formula mass (M r ) of the compound.Īdd up the atomic masses of the atoms in the empirical formula.įor example, the empirical formula of a hydrocarbon is CH 2 and its M r is 42. In this example, you would multiply both numbers by 2, giving 2 and 3 (instead of rounding 1.5 up to 2). Sometimes it does not, so you might get 1 and 1.5. To determine the molecular formula we will use the empirical formula. The action at Step 5 usually gives you the simplest whole number ratio straightaway. The molar mass was determined to be 180.16 g/mol. What is the formula of the oxide?įor your calculation you’ll need to use the fact that the A r (relative atom mass) of sulfur is 32 and the A r of oxygen is 16. The formulas to keep in mind are: multiple of empirical formula molecular mass /. Here is an example:ģ.2 g of sulfur reacts with oxygen to produce 6.4 g of sulfur oxide. Multiply each subscript in the empirical formula by the answer in step 2. You can use information about reacting masses to calculate the formula of a compound. Step 2: Divide molar mass of BH 3 with the empirical formula mass.

Step 1: Calculate empirical formula mass (EFM) E.F.M of BH 3 13.84g/mol.

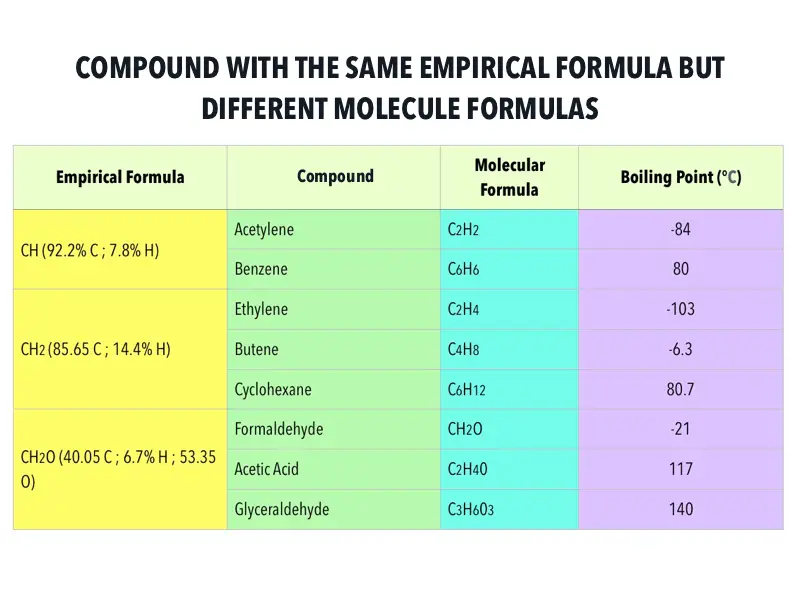
Determine the molecular formula of the compound. On the other hand, a compound which has the empirical formula of CH 2 could have a molecular formula of C 2 H 4, C 3 H 6, C 4 H 8 or even C 13 H 26. EXAMPLE: The empirical formula of a compound of boron and hydrogen is BH 3. This is because we can divide each number in C 6 H 12 O 6 by 6 to make a simpler whole number ratio. įor example, the molecular formula of glucose is C 6 H 12 O 6 but the empirical formula is CH 2 O. Molecular Formula calculator uses Molecular Formula Molar Mass/Mass of Empirical Formulas to calculate the Molecular Formula, Molecular Formula is the. It is determined using data from experiments and therefore empirical. The empirical formula of a compound is the simplest whole number ratio of atoms of each element in the compound.


 0 kommentar(er)
0 kommentar(er)
